Our Quasi-Favorskii Rearrangement highlighted in Organic Letters front cover
/Work from Nicole, Stephen and Juha made it to the front cover of Organic Letters issue 15/2020. Great job! See the full publication here.
Our Nature Catalysis Article is Featured in Rice News!
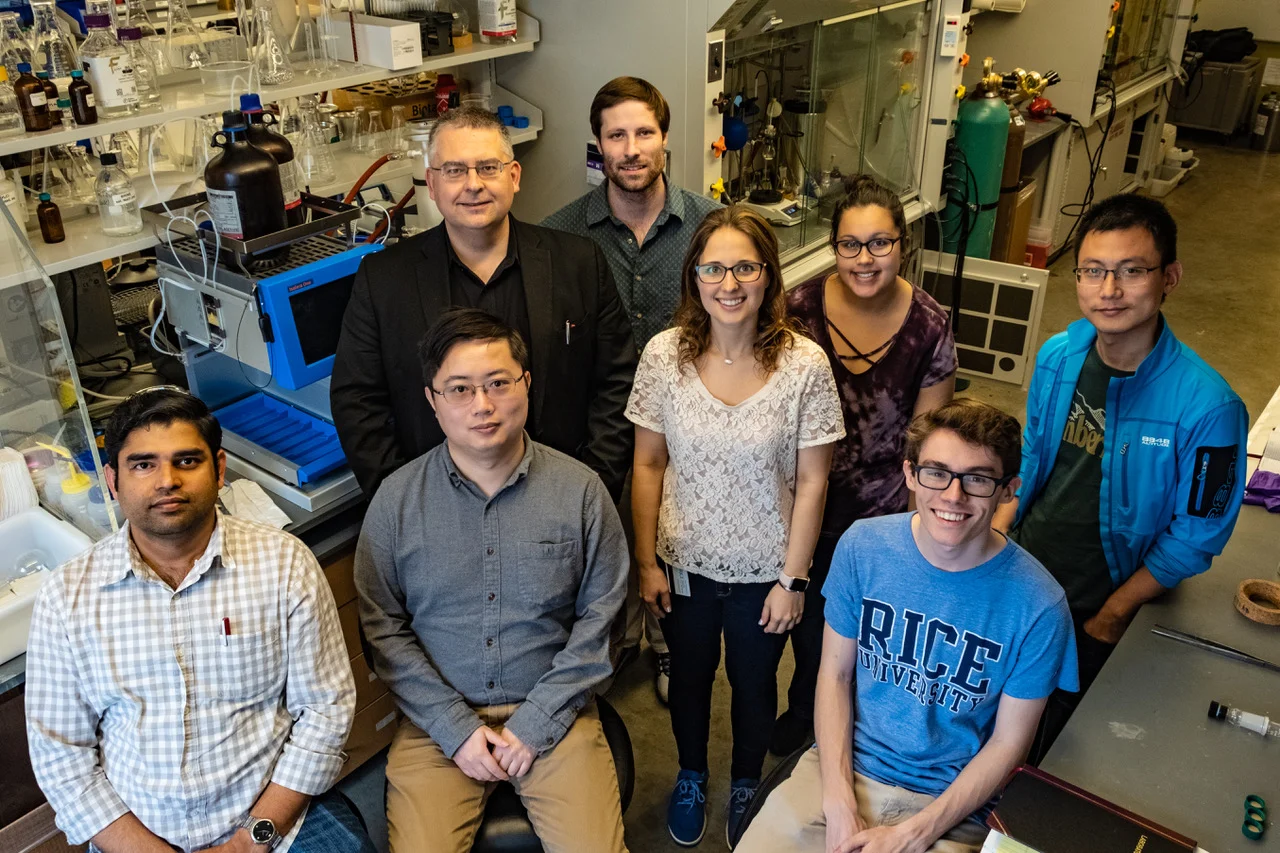
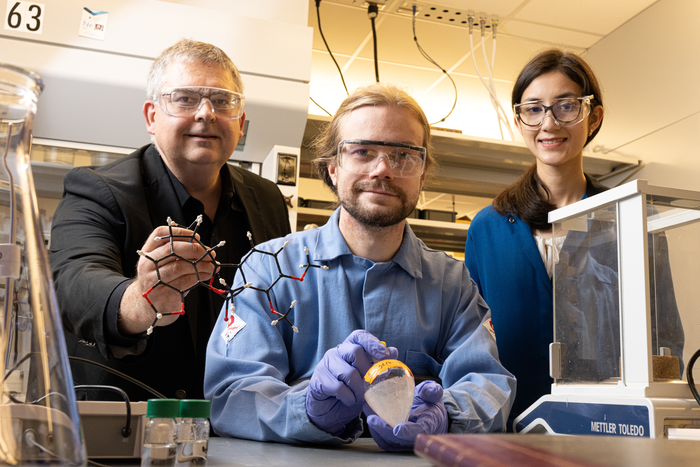
/Rice researchers have further simplified their process to make essential precursor molecules for drug discovery and manufacture. From left: Zhe Zhou, Juha Siitonen, Qing-Qing Cheng (on screen) and László Kürti. Courtesy of the Kürti Research Group
Photo Credit: Mike Williams
Our JACS communication on aza-Rubottom oxidation is featured in Rice News!
/Photo Credit: Jeff Fitlow
Nitrogen gets in the fast lane for chemical synthesis
Congratulations to ZZ and Qing!
Nicole and Kaitlyn's "Fun with Chemistry" Outreach Program is featured in "Rice at Large"
/In 2017, group members Nicole Behnke and Kaitlyn Lovato started the first extension chapter of Fun with Chemistry at Rice University, an outreach program that uses exciting and educational experiments to inspire K–12 students to pursue careers in STEM.
The Fun with Chemistry pro- gram was founded in 2014 by Kate Biberdorf, a chemistry lecturer at the University of Texas at Austin. After working with Biberdorf as an undergraduate, Nicole Behnke joined the Rice chemistry department for her graduate studies and started a collaboration to expand Fun with Chemistry. László Kürti, associate professor of chemistry at Rice, is the faculty sponsor. Kürti and Biberdorf secured a $75,000 Welch Foundation grant to support outreach events for both Rice and UT over three years.
The Rice University Fun with Chemistry chapter is managed by graduate students Nicole Behnke, Kaitlyn Lovato and Katie Miller with the help of graduate and undergraduate student volunteers.
The mission of Fun with Chemistry is to ignite, inspire and motivate young students to develop a passion for science with the hope that they will pursue careers in STEM. To do this, the program introduces educational topics and then conducts hands- on experiments that reinforce and elaborate on the lesson. The program is designed to appeal to students who are intimidated by science or attend schools that do not have the resources to con- duct captivating experiments. Specifically, the program targets schools with high minority and disadvantaged socioeconomic populations.
More at: https://publicaffairs.rice.edu/sites/g/files/bxs501/f/pdf/ral/RALwinter19.pdf
Rice Chapter “Fun with Chemistry” Website: https://www.ricefunwithchem.com
The group says farewell to Dr. Zhiwei Ma.
/Dr. Zhiwei Ma has taken a position of Research Investigator at the Genomic Institute of Novartis Research Foundation (GNF) in San Diego, CA. Congratulations and best wishes!
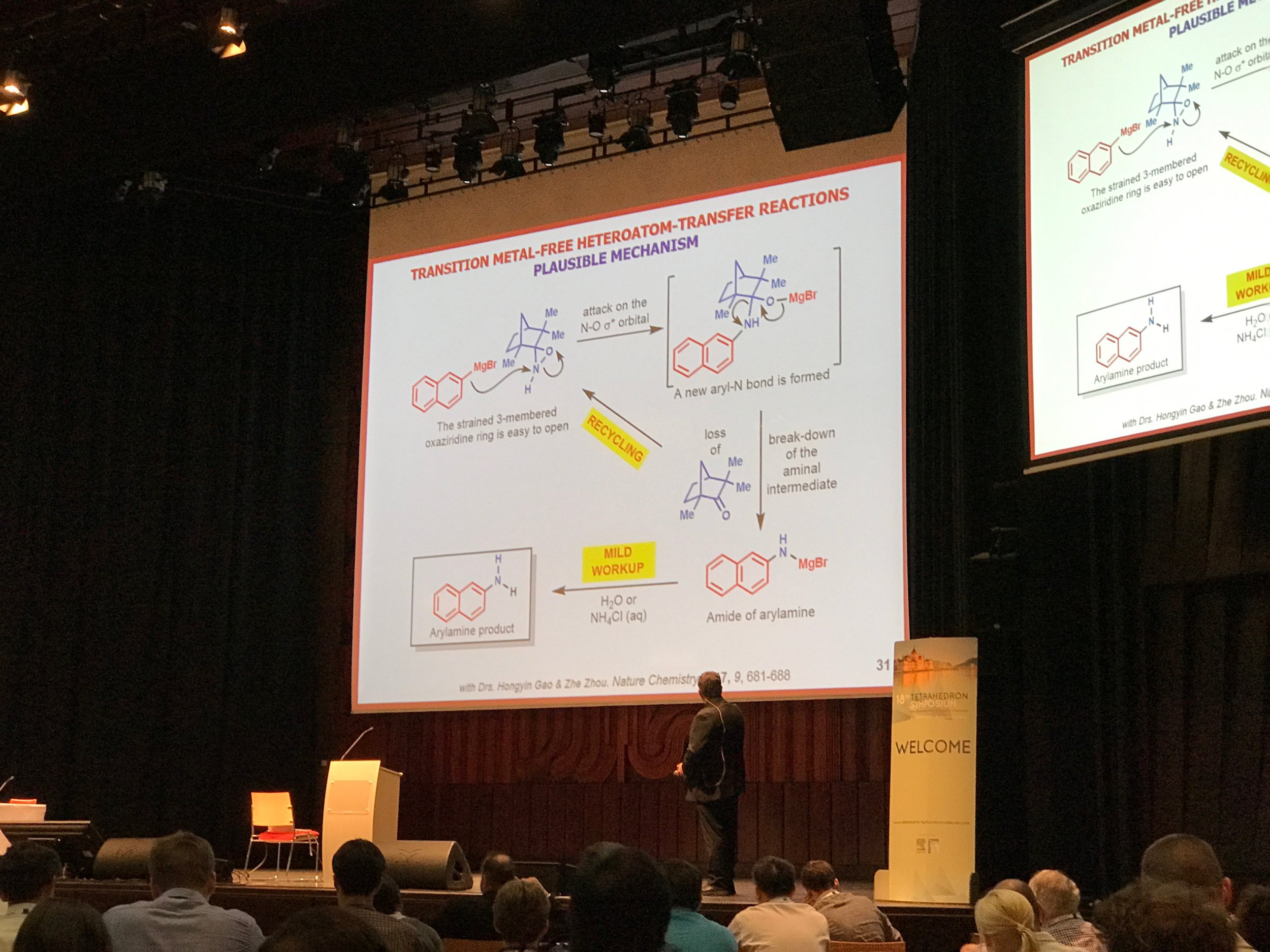
Kürti Group Members Attended the 18th Tetrahedron Symposium in Budapest
/Group members Dr. Zhe Zhou, Miss Nicole Behnke and Miss Kaitlyn Lovato presented their research at the 18th Tetrahedron Symposium in Budapest, Hungary. Professor Kürti was a invited keynote speaker for this high profile conference and gave a talk on the group's research.



Kürti Group Members Won Fellowship Awards!
/Graduate student Miss Kaitlyn Lovato was awarded the prestigious NSF Graduate Research Fellowship (September 1st 2017 - September 1st 2020).
Undergraduate student Mr. James Siriwongsup was awarded the George Holmes Richter Fellowship in Chemistry, a 10-week fellowship in support of undergraduate chemistry summer research.
Undergraduate student Mr. Russell Kielawa was awarded the Zevi & Bertha Salsburg Memorial Fellowship in Chemistry.
Congratulations to Kaitlyn, James and Russell!
Two of our recent papers are highlighted by Rice News!
/Rice News highlighted two of our recent publications. Congratulations to Venkatesh, Zhiwei, Zhe, Jun and James! Special thanks to Mr. James Siriwongsup, who contributed a lot to a high impact project as an undergraduate student!
Rice scientists simplify the incorporation of nitrogen into molecules
Greener molecular intermediates may aid drug design
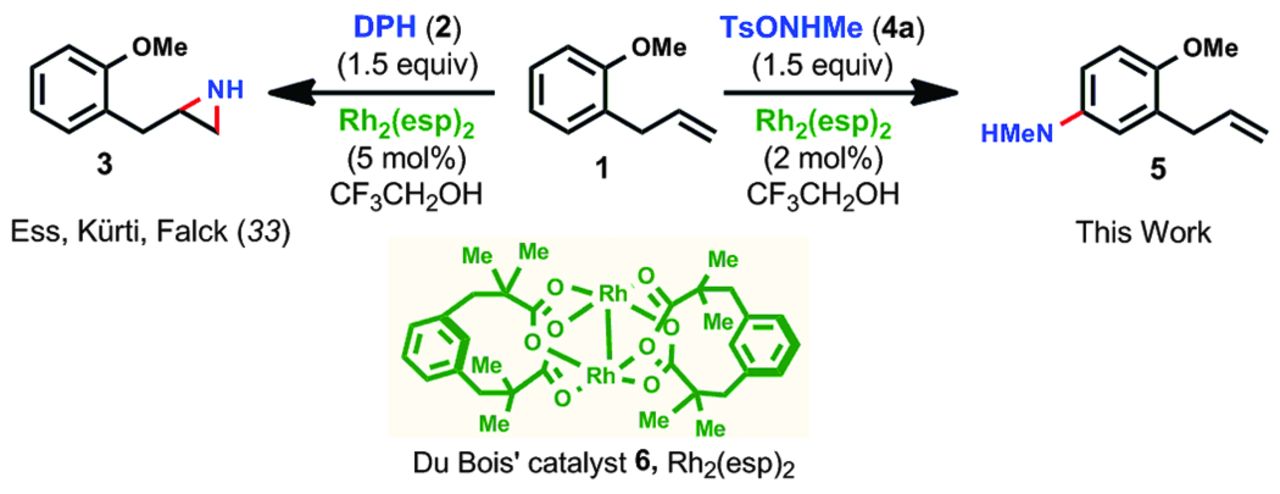
Our Research is highlighted by C&EN
/Dr. Kattamuri's recent JACS paper has been highlighted by C&EN! This project is a collaboration between the Kürti group at Rice, the Ess group at Birgham Young and the Li group at Nanjing University/Texas Tech. The newly developed method can make secondary aryl amines without the use of transition-metal catalysis.
Learn more at: http://cen.acs.org/articles/95/i28/Arylamines-made-easy.html
Our Research is published in Nature Chemistry!
/The Rice lab of synthetic chemist László Kürti reported its success at creating highly efficient aminating and hydroxylating reagents from abundant and biorenewable terpenoids that promises to make the reagents' use environmentally friendly and cost-effective.
Read the abstract at http://dx.doi.org/10.1038/nchem.2672
News release can be found online at http://news.rice.edu/2016/11/28/pine-product-offers-fresh-take-on-fine-chemical-synthesis/
Our collaborative Science paper just went online!
/Primary and N-alkyl arylamine motifs are key functional groups in pharmaceuticals, agrochemicals, and functional materials, as well as in bioactive natural products. However, there is a dearth of generally applicable methods for the direct replacement of aryl hydrogens with NH2/NH(alkyl) moieties. Here, we present a mild dirhodium-catalyzed C-H amination for conversion of structurally diverse monocyclic and fused aromatics to the corresponding primary and N-alkyl arylamines using NH2/NH(alkyl)-O-(sulfonyl)hydroxylamines as aminating agents; the relatively weak RSO2O-N bond functions as an internal oxidant. The methodology is operationally simple, scalable, and fast at or below ambient temperature, furnishing arylamines in moderate-to-good yields and with good regioselectivity. It can be readily extended to the synthesis of fused N-heterocycles. (Link to the article: DOI: 10.1126/science.aaf8713)
See press release here: http://news.rice.edu/2016/09/12/chemists-make-strides-to-simplify-drug-design-and-synthesis/
Kurti Group's New Website
/Welcome to our brand new group website, stay tuned for additional content!